Abstract
Background:Since their approval in 2003, Tyrosine Kinase Inhibitors (TKIs) targeting bcr-abl1 kinase activity have changed the treatment paradigm for children with chronic myeloid leukemia (CML) and Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL). However, off-target inhibition of kinases, such as src and c-kit, can result in impaired osteoblast function, cause an imbalance in calcium and phosphate metabolism, and result in growth impairment in children. The aim of our study was to examine growth over time in pediatric patients receiving TKIs for CML or Ph+ ALL.
Description: This is a single institutional retrospective longitudinal cohort study of patients (≤ 21 years of age) diagnosed with CML or Ph+ ALL between January 2000 and December 2015 who received a TKI and had ≥ 1 year of growth data available as of May 31, 2016. Patient demographics, treatment details including the type and duration of TKI therapy and details of concomitant conventional therapy (chemotherapy and radiation) were abstracted. Growth data was abstracted from the start of TKI at yearly intervals up to a maximum of five years or age 20 and from their last available appointment. Specific variables for growth included height, height percentile, height-SDS (height - standard deviation score or z score) and BMI-SDS (body mass index - standard deviation score). Height-SDS and BMI-SDS were determined using the CDC growth chart by age and gender. Age at diagnosis was used as a surrogate for expected pubertal status: pre-pubertal (3 - 8.9 years), pubertal/peri-pubertal (9 - 15.9 years), post-pubertal (16+ years). Overall change in height-SDS / BMI-SDS from baseline to 1-year and last follow-up were compared by diagnosis, gender, and pubertal age using ANOVA. Statistical analysis was performed using SAS® v9.3 (Cary, NC). For all analyses, a p-value of <0.05 was considered to be statistically significant.
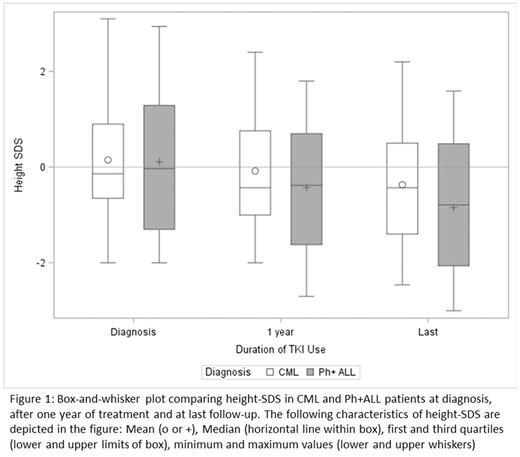
Results: Our cohort included 48 patients who received a TKI (n=29 CML, n=19 Ph+ ALL) - 46% were male and 38% were non-Hispanic white. The mean age at diagnosis was 12.1 ± 3.7 years for CML and 9.0 ± 4.6 years for Ph+ ALL patients (p=0.01). As expected, mean duration of TKI treatment was longer in CML (4.3 ± 2.8 years) versus Ph+ ALL (2.7 ± 1.7 years). The average duration of follow-up was similar in both groups (CML 4.6 ± 3.54 years vs Ph+ ALL 4.1 ± 2.9 years, p=0.60). At time of diagnosis, the mean Height-SDS for the entire cohort was 0.13 ± 1.28 with no difference between patients by diagnosis (p=0.92). At one year following treatment with TKI a decrease in mean height-SDS was noted, with a more significant difference in Ph+ ALL patients (-0.54 ± 0.21) compared to CML patients (-0.18 ± 0.39) (p=0.002). This difference remained significant at last follow-up (Ph+ ALL -0.96 ± 0.70, CML -0.52 ± 0.74, p=0.05) (Figure 1). There was no significant difference in mean change in height-SDS at 1 year or last follow-up by gender. Overall, the decrease in height-SDS at 1 year was most significant in the pre-pubertal age group (-0.51 ± 0.27) as compared to pubertal/peri-pubertal and post-pubertal groups (-0.26 ± 0.46 and -0.10 ± 0.24 respectively; p=0.04). BMI-SDS at baseline did not differ by diagnosis but did show a significant decrease at one year in Ph+ ALL versus CML patients (-0.18 ± 0.65 and 0.39 ± 0.54 respectively, p=0.003); this stabilized at the time of last follow up (-0.01 ± 0.75 and 0.38 ± 0.80 respectively, p=0.11).
Conclusions: Our data underscores the concern of growth disturbances following prolonged TKI exposure in children, the role of exposure relative to pubertal change in bone growth, and the need for understanding off-target mechanisms in childhood survivors. Stabilization of BMI-SDS at the end of follow up with accompanying drop in mean height-SDS points to a mechanism of growth disturbance beyond nutritional deficiencies. This large pediatric cohort treated with TKIs is the first to include Ph+ ALL patients who were younger at onset of TKI treatment, and more likely to be pre/peripubertal at the start TKI exposure. Future directions will focus on a prospective study of musculoskeletal and other organ toxicities in patients treated with TKIs in childhood.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal